KINUGASA REACTION AS A STEREOSELECTIVE METHOD OF β-LACTAMS SYNTHESIS: ADVANCED APPLICATIONS
β-Lactam ring is a key structural element of vast pharmacologically active compounds, including penicillins, cephalosporins, carbapenems, nocardicines or modern cholesterol lowering agents (e.g. ezetimibe). Bioactivity of β-lactamic derivatives is a driving force for search of new bioactive compounds, as well as, for the development of new strategies of the 2-azetidinone ring formation.
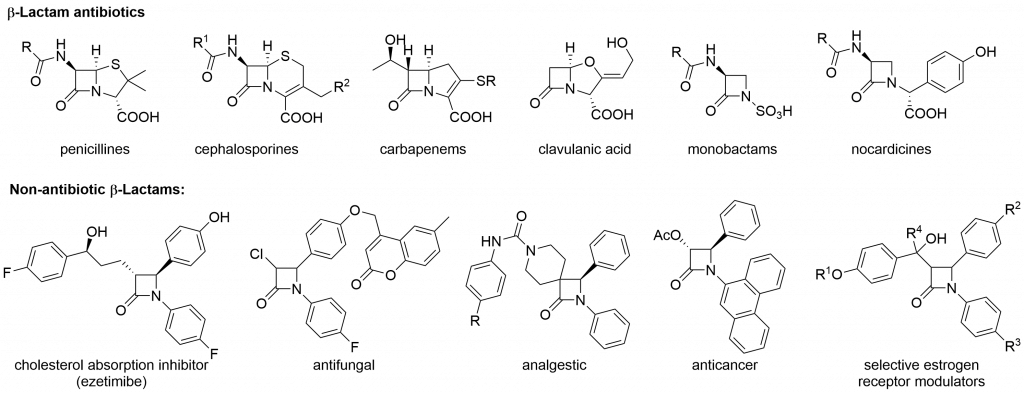
One of the rarely used method of β-lactams formation is Cu(I)-catalyzed reaction of terminal alkynes and nitrones (Kinugasa reaction).
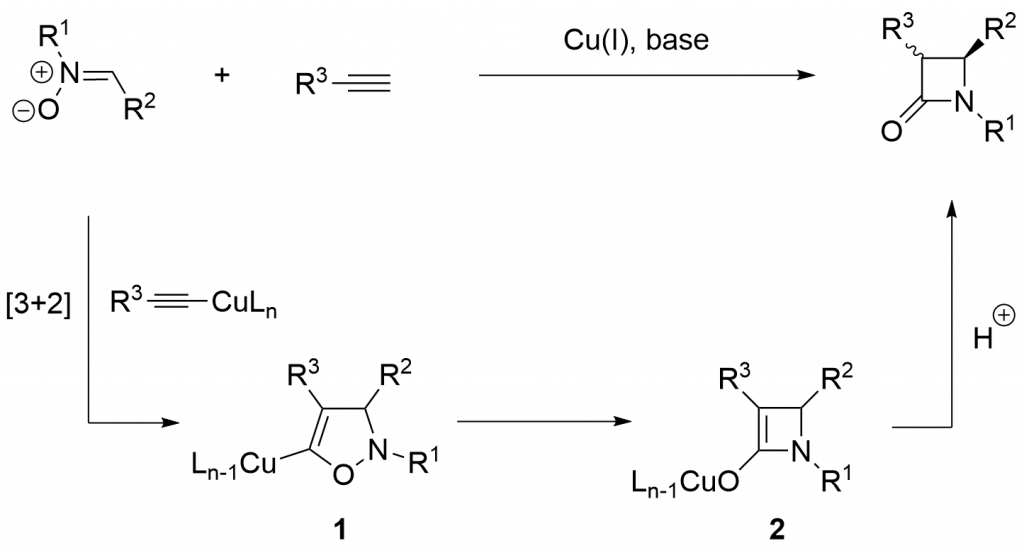
Although the Kinugasa reaction was discovered 30 years ago, it received more attention in last years. Significant part of this come-back is due to our pioneering works which have been initiated several years ago. Over the years, we demonstrated that bicyclic framework of carbapenam antibiotics can be obtained efficiently and stereoselectively by the reaction of terminal alkynes and chiral cyclic nitrones.1-5 Also, the approach was used to construct of monocyclic azetidinones recently.6,7 The last achievement was intramolecular variant of Kinugasa reaction.8 The practical application of this part of our work represents the use of Kinugasa in the synthesis of ezetimibe (commercially available cholesterol absorption inhibitor)9, Kaneka β-lactam10 (chiral industrial precursor of synthetic carbapenems), and elaboration of a strategy leading to thienamycin11,12 and related antibiotics.
[1] Stecko, S.; Mames, A.; Furman, B.; Chmielewski, M., J. Org. Chem. 2008, 73, 7402-7404;
[2] Stecko, S.; Mames, A.; Furman, B.; Chmielewski, M., J. Org. Chem. 2009, 74, 3094-3100;
[3] Mames, A.; Stecko, S.; Mikołajczyk, P.; Soluch, M.; Furman, B.; Chmielewski, M., J. Org. Chem. 2010, 75, 7580-7587;
[4] Woźnica, M.; Masnyk, M.; Stecko, S.; Mames, A.; Furman, B.; Chmielewski, M.; Frelek, J., J. Org. Chem. 2010, 75, 7219-7226;
[5] Kabala, K.; Grzeszczyk, B.; Stecko, S.; Furman, B.; Chmielewski, M., J. Org. Chem. 2015, 80, 12038–12046.
[6] Kabala, K.; Grzeszczyk, B.; Furman, B.;,Chmielewski, M.; Solecka, J.; Guśpiel, A. Synthesis, 2018, 50, 1991-2000
[7] Kutaszewicz, R.; Grzeszczyk, B.; Górecki, M.; Staszewska-Krajewska, O.; Furman, B.; Chmielewski, M., Org. Biomol. Chem., 2019, 17, 6251-6268
[8] Popik, O.; Grzeszczyk, B.; Staszewska-Krajewska, O.; Furman, B.; Chmielewski, M., Org. Biomol. Chem., 2020, in press
[9] Michalak, M.; Stodulski, M.; Stecko, S.; Mames, A.; Panfil, I.; Soluch, M.; Furman, B.; Chmielewski, M., J. Org. Chem. 2011, 76, 6931-6936.
[10] Grzeszczyk, B.; Stecko, S.; Mucha, L.; Staszewska-Krajewska, O.; Chmielewski, M.; Furman, B., J. Antibiot. 2013, 66, 161-163.
[11] Soluch, M.; Grzeszczyk, B.; Staszewska-Krajewska, O.; Chmielewski, M.; Furman, B., J. Antibiot. 2016, 69, 164-168;
[12] Pieczykolan, M.; Furman, B.; Chmielewski, M. J. Antibiot. 2017, 70, 781-787.
