STUDIES ON THE REARRANGEMENT OF VINYL ETHERS AND ALKOXYDIENES
The formation of C-C bond is one of fundamental transformation of organic chemistry. Particularly important and highly desired are methods that allow for formation of new C-C bond in highly stereoselective manner. The Claisen rearrangement is one of classical synthetic method that enables this kind of chemical transformation via [3,3] sigmatropic rearrangement. Another powerful, but less popular, method of construction of a new C-C bond by the breaking C-O one, is [1,3] rearrangement reaction of vinyl ethers (O to C rearrangement). The molecules, bearing latent electrophilic and nucleophilic moieties rearrangement to the product by formation of a new carbon-carbon bond. The most prevalent oxygen-to-carbon rearrangements are those whereby the positive charge at the electrophilic species can be stabilized by heteroatom, for instance oxygen.
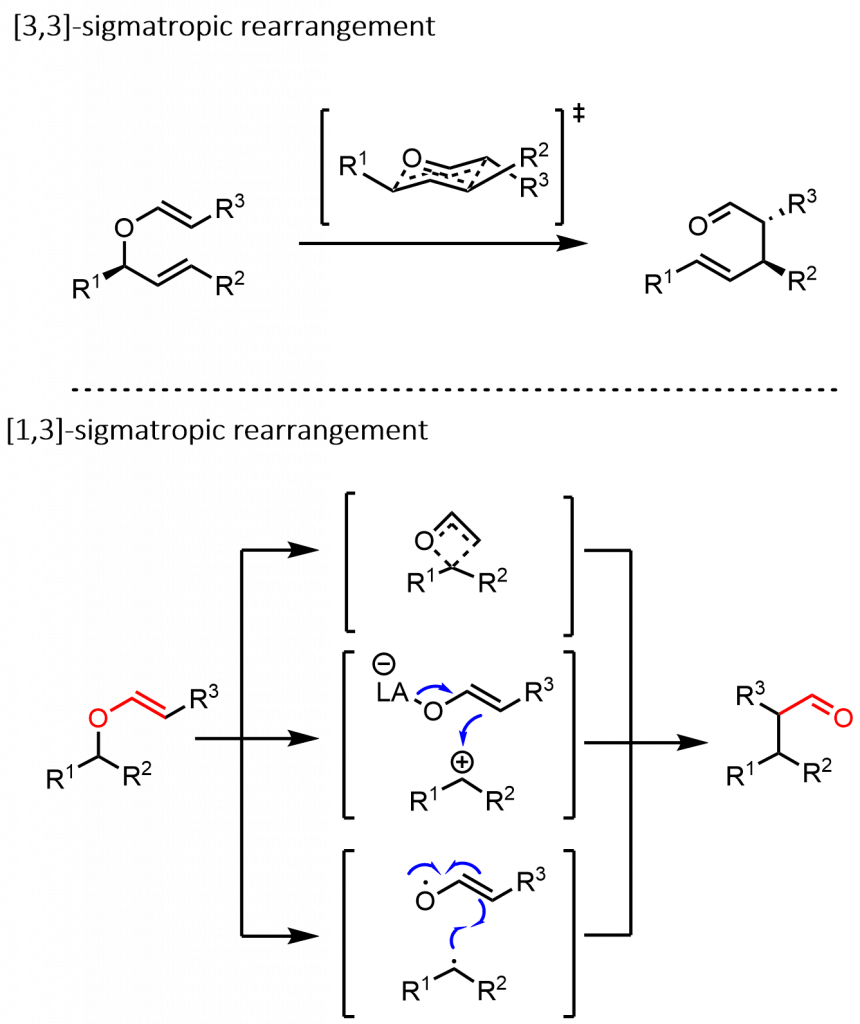
Our group has very recently discovered that substituted vinyl ethers and unsymmetrical ketene acetals undergo smooth conversion to chain-extended ketones or esters with a catalytic amount of trimethylsilyl triflate.1
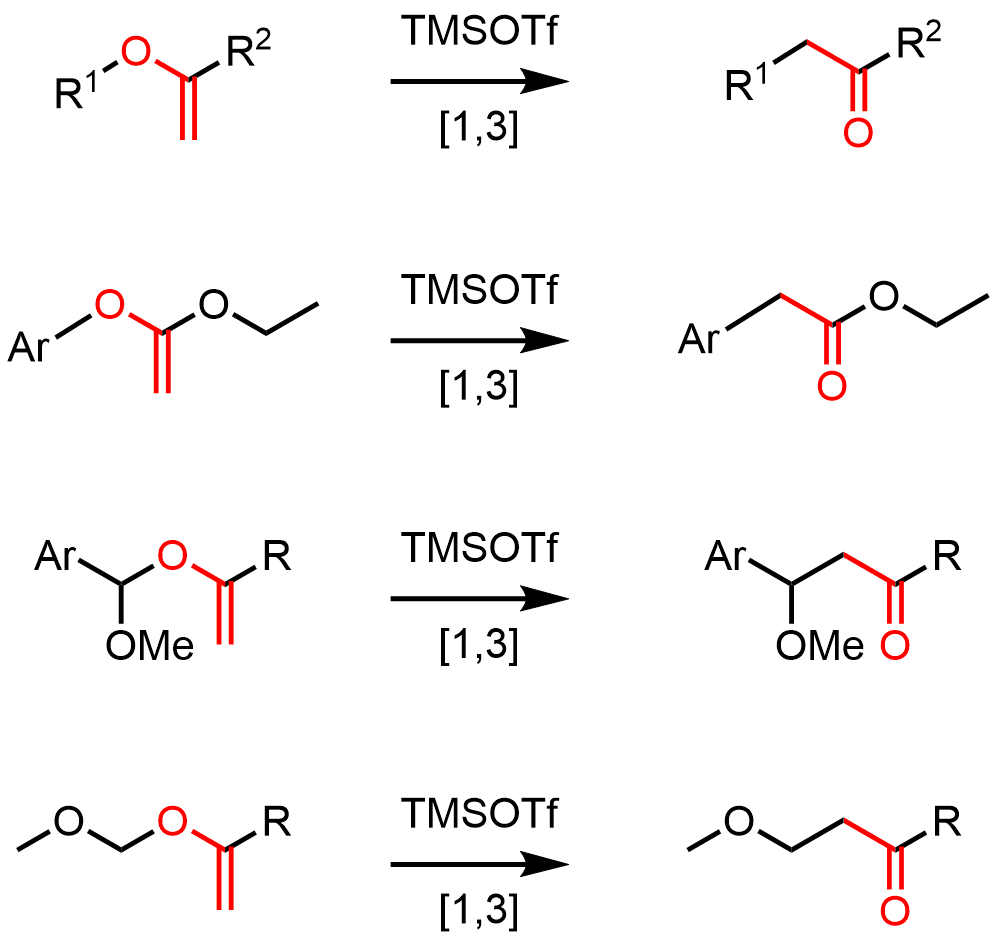
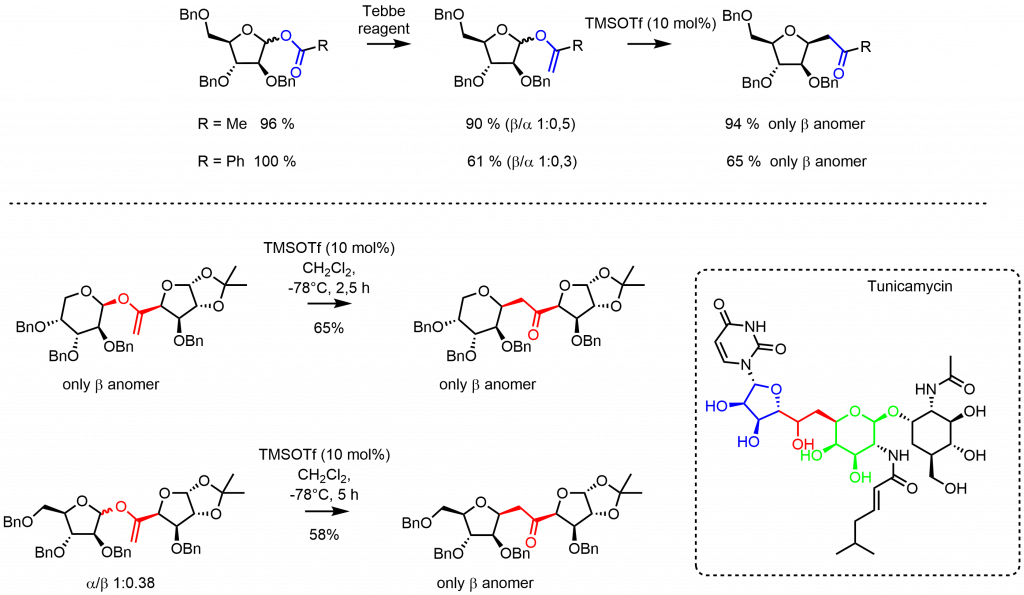
Further examination showed that the developed reaction conditions can be applied to the transformation of sugar-derived anomeric vinyl ethers. This highly stereoselective transformation opens an access to C-glycosides and C-disaccharides – important, acid hydrolysis-resistant C nucleoside analogues.
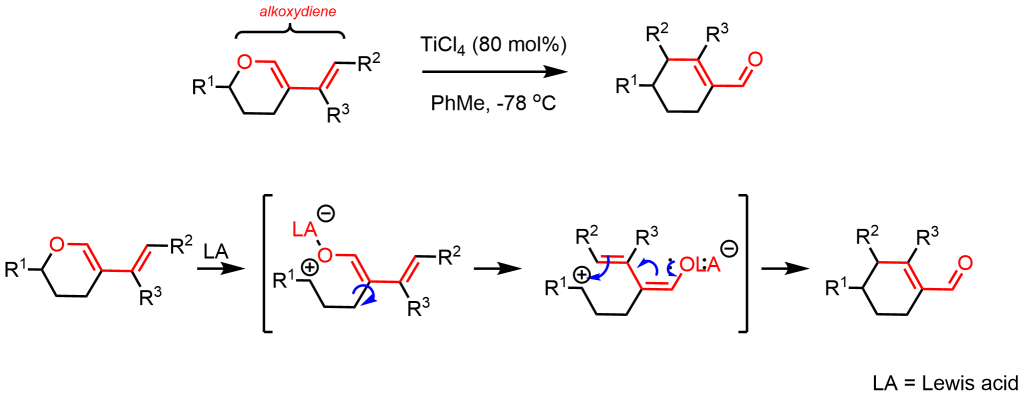
Recently, we have found that O-1,3-alkoxydienes undergo a smooth vinylogous Ferrier-Petasis-type reaction when treated by a catalytic amount of titanium(IV) chloride.2 This rearrangement reaction proceeds with good chemical yield and regioselectivity to afford the corresponding cyclohexene carbaldehydes which are important structural synthons for the synthesis of numerous biologically active molecules.
Our the most recent results allow to direct, effective, and operationally simple transformation of esters into ketones or alkenes by the exclusive action of Tebbe’s reagent.3 The transformation utilizes the dual character of Tebbe’s reagent as both a methylenation agent and a rearrangement catalyst in the reaction of a wide range of substituted vinyl ethers. The resulting transformation involves sequential methylenation and rearrangement reactions and it offers a high degree of selectivity toward the synthesis of ketones or alkenes.
[1] E. Maziarz, B. Furman, Tetrahedron 2014, 70, 1651-1658
[2] A. Domżalska, E. Maziarz, B. Furman, Tetrahedron 2017, 73, 7030-7041
[3] A. Domżalska, B. Furman, Synlett 2020, 31, 730-736
